Cooperative Locations
Cooperative Organ Cancer Centres
A cooperative Organ Cancer Centre (e.g. breast, colon) is a union of several (clinics-) locations in a centre, which provides a basic care for oncology patients specific to the organ, appropriate to the requirements. Basic care includes especially diagnostic therapy (examination), surgery and inpatient treatment.
Partnership instead Cooperation
The establishment of cooperation centres is often underestimated by participants, in terms of effort and requirements. Basically, cooperations should be an exception, especially when the participating hospitals have different owners. Generally, a collaboration between centres is possible (e.g. common tumour boards) even without starting a cooperation.
Requirements for cooperating Organ Cancer Centres
The purpose of a cooperative Organ Cancer Centre is a comparable patient care, in terms of method and quality. This means that the patients, can expect this comparable care regardless the hospital-location. A cooperating location has to prove entirely also the fulfilment of the requests of certification.
Simultaneous initial certifications of multiple locations
The simultaneous initial certifications of several locations shall be avoided. In the case of cooperating locations, an already successfully certified centre is usually extended with a new location. A simultaneous initial certification is possible only in case of very positive structural conditions (including identical carriers).
Structural requirements
For the structural design of Organ Cancer Centres the document “Basic information for certification” contains important indications and structural requirements. This document has to be analysed in detail as soon as possible in planning an Organ Cancer Centre and has a great importance for the cooperating locations. The integration of OnkoZert in planning a cooperative centre also needs to take place very soon. It must be definitely prevented that in a moment in which many preliminary works have already been carried out, to question the principle structure of the cooperating centre.
Evaluation of structure
Each cooperation requires a written assessment of the structure, which will decide on the admission of cooperation (subject to fees). Evaluating the structure is a costly and time consuming process, in which the requirements for the structural and central certification will be well analysed and evaluated. For the evaluation of the structure there are special templates, that will be asked for directly from OnkoZert.
Guidelines for evaluation of structure
The evaluation of the structure conducted by the Certificate Award Committee will be made, among the guidelines presented below. These guidelines do not represent a criterion for exclusion from the admission to the certification procedure. In justified exceptional cases, the Certificate Award Committee may issue a special admission, in connection with certain requirements.
| Holder | A common holder of cooperating locations is an advantage. |
| Distance | The distance of the locations compared to the main location must not exceed 45 km, respectively a route of 45 min. (calculated by Google Maps – the fastest route). |
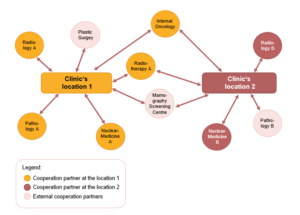
| Number of the cooperating locations | Each additional location increases the requirements and costs of a collaboration. Exceptions may be locations that are under the same owner and that are disciplinary driven by a medical leadership. – 2 locations: Has been proven in some cases as a significant and useful structure for all parties involved. – 3 locations: Can be difficult and to the extent possible, not be regarded as a preferred embodiment. If certain aspects are taken into consideration, this model can still be advantageous. – 4 locations: Request in terms of coordination extreme claims, therefore it is generally not recommended. – More than 4 locations: A cooperation centre with more than 4 locations is considered to be highly critical based on actual experiences and it is absolutely not recommended. |
| Number of primary cases | Each location of a cooperative centre must independently prove the case / primary case requirements. With regard to exemptions in the area of Breast (preservation of the status quo) and Lung (continuity of the centre management) OnkoZert is to be contacted directly. |
| Type of QM (quality management) system | DKG-certified centres should implement a certified QM system, but there is no obligation to certify a QM system. Different types of QM systems at the locations are disadvantageous (e.g., 2 KTQ sites and one ISO 9001 site). If the cooperating centre consists of more than 2 locations, it is advisable to have a cross-location QM system in which the cooperation between the locations is regulated and controlled. |
| Cooperation partner | The more integrated cooperation partners a centre has, the more difficult the coordination in the centre and between the cooperation partners of a discipline is. |








